
A Certificate/Diploma/Professional Development Program in Clinical Research Methodology is a focused educational pathway designed to equip individuals with the essential knowledge and skills Clinical research training programs required to effectively/successfully/proficiently conduct clinical research studies. This comprehensive program covers/delves into/explores a wide range/various aspects of clinical research, including study design, protocol development, data management/analysis/collection, regulatory compliance, and ethical considerations. Upon successful completion/graduation/achievement of the program, participants will gain/acquire/develop a solid understanding of the principles and practices underlying clinical research, preparing them for rewarding careers in this dynamic/growing field.
- Participants/Learners/Students will develop expertise in designing and implementing clinical trials
- Graduates of the program will become familiar with relevant regulatory guidelines and ethical standards
- This certificate program offers a flexible learning format to accommodate working professionals
In-Depth Training in Clinical Trials Management
A well-rounded curriculum is essential for effective clinical trials management. This training should encompass a broad range of areas, from study development to data analysis. Students will gain essential knowledge in regulatory guidelines, medical best practices, and project coordination. A practical approach is crucial to provide that trainees are thoroughly ready to navigate the complexities of clinical trials.
After of this program, participants will be competent to successfully oversee all aspects of a clinical trial, promoting the advancement of new and groundbreaking therapies.
Essentials of Good Clinical Practice (GCP) Certification
Obtaining certification in Good Clinical Practice (GCP) is crucial for experts involved in the design, conduct, and reporting of clinical trials. This certification demonstrates a deep understanding of ethical principles, regulatory guidelines, and best practices within the field. GCP certification ensures that clinical trials are conducted ethically, safeguarding participant welfare and producing reliable data for pharmaceutical advancements.
The rigorous curriculum encompasses a range of areas, including informed consent, information management, monitoring and auditing, patient welfare, and GCP regulatory requirements. Successful completion typically involves both theoretical knowledge and practical tests.
- Participants seeking GCP certification often include: clinical research associates, physicians, pharmacists, nurses, data managers, regulatory affairs specialists
The value of GCP certification is widely recognized across the biotechnology industry. Employers require qualified professionals who display a commitment to high ethical and quality standards in clinical research.
Excelling in Clinical Data Management and Analysis
In the realm of healthcare research, efficient clinical data management and analysis are essential for producing reliable findings. Effective data management involves a multifaceted approach that encompasses collection, storage, and processing of patient data in a compliant manner. This promotes the validity of data across the research process.
Furthermore, skilled analysts employ statistical tools to reveal meaningful patterns within the data. Such interpretations offer valuable data for research decision-making and progressing patient care.
Cutting-Edge Clinical Research Design and Execution
Conducting rigorous and clinical research necessitates a meticulous approach to both design and execution. This involves rigorous planning at each stage, from the initial idea to the conclusive analysis. Researchers must precisely select study participants, formulate appropriate endpoints, and implement robust information collection methods. Moreover, adherence to stringent ethical guidelines is critical throughout the research process.
- Effective clinical research design requires a deep understanding of both clinical principles and data-driven methodologies.
- Execution of clinical trials demands collaboration among diverse stakeholders, including researchers, participants, regulatory agencies, and biotechnology companies.
Connecting the Gap: From Bench to Bedside - Clinical Research Training
Clinical research stands as a vital foundation of healthcare advancement. It's the pivotal link between groundbreaking discoveries in the laboratory and their practical application in treating patients. However, translating scientific findings into effective treatments can be a complex and challenging process. This is where robust clinical research training comes into play.
- Clinicians, researchers, and experts involved in clinical trials need specialized knowledge and skills to plan rigorous studies, acquire reliable data, and evaluate results with accuracy.
- Furthermore, effective communication and collaboration between diverse members are essential for the effective translation of research findings into tangible clinical benefits.
Clinical research training programs equip individuals with the necessary tools and expertise to bridge this gap, ensuring that scientific progress directly translates into improved patient outcomes.
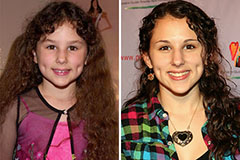 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Mackenzie Rosman Then & Now!
Mackenzie Rosman Then & Now!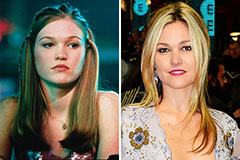 Julia Stiles Then & Now!
Julia Stiles Then & Now! Traci Lords Then & Now!
Traci Lords Then & Now! Katey Sagal Then & Now!
Katey Sagal Then & Now!